Treatment Manuals
ALACRITY: Treatment Development and Deployment Model
ALACRITY developed a novel model of deployment-based behavioral interventions and implementation, streamlined based on neurobiology models and augmented by mobile technology. Rather than focusing exclusively on uptake and sustainability of available interventions, many of which are too complex for community use, ALACRITY investigators are working to both simplify the treatments themselves and improve their delivery. Accordingly, our model:
1) Targets groups identified by consumers, community partners, and our team.
2) Develops its interventions jointly with community partners and a transdisciplinary team and uses neurobiological concepts as a “simplification rule” for streamlining behavioral interventions so that they can be used by community clinicians;
3) Integrates mobile technology to community interventions at the assessment, the intervention, and the adherence monitoring levels; and
4) Tests its interventions at community sites using community clinicians to shorten the way to uptake and sustainability.
To maximize the impact of its treatments, ALACRITY investigators work both in settings in which most older and middle-aged people receive care (primary care) and in settings serving persons with special clinical (elder mistreatment) and social needs (poverty). Our approach is a clear departure from traditional intervention and services research and can lead to a revolutionary change in how the field thinks about T2 intervention development and deployment in the community.
ALACRITY: Developmental Project RELIEF
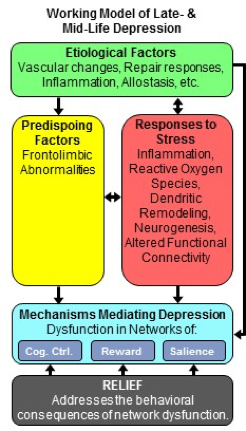
This developmental project combines the expertise of our Cornell transdisciplinary team and of our community-based, primary care partners. We propose to test the reach, feasibility, acceptability, and preliminary effectiveness of Relief, a 9-session behavioral intervention for older and middle-aged primary care patients with chronic pain and depression. Relief is designed to be administered by licensed social workers (LCSWs) and/or nurse practitioners (NP) of primary care practices.
Our conceptual model assumes that chronic pain and depression are characterized by an attentional bias assigning greater salience to interoceptive stimuli and to negative emotions, along with a difficulty in shifting attention to goal-oriented/reward-driven state, leading to inadequate engagement of the reward networks. Accordingly, Relief aims to shift patient attention away from pain and negative feelings and to increase their focus and engagement in meaningful and pleasurable activities. Relief also assesses patient views on pain treatment, which may be adversely affected by depression, corrects unrealistic expectations, and helps to enhance communication between patients and primary care physicians. To improve its delivery and enhance behavioral assessment, Relief uses easy-to operate smartphone apps.
This project will assess Relief’s reach, feasibility, and acceptability; evaluate preliminary effectiveness; assess preliminary evidence of target engagement; and perform exploratory analyses on sex differences, quality of life, pain medication use, suicidal ideation,cost and savings. We will use the results of this project to support an R01 application to rigorously test the feasibility, acceptability, effectiveness, non-billable costs and savings, and barriers to implementation of Relief in a randomized effectiveness trial.Our conceptual model assumes that chronic pain and depression are characterized by an attentional bias assigning greater salience to interoceptive stimuli and to negative emotions, along with a difficulty in shifting attention to goal-oriented/reward-driven state, leading to inadequate engagement of the reward networks. Accordingly, Relief aims to shift patient attention away from pain and negative feelings and to increase their focus and engagement in meaningful and pleasurable activities. Relief also assesses patient views on pain treatment, which may be adversely affected by depression, corrects unrealistic expectations, and helps to enhance communication between patients and primary care physicians. To improve its delivery and enhance behavioral assessment, Relief uses easy-to operate smartphone apps.
ALACRITY: Developmental Project PROTECT
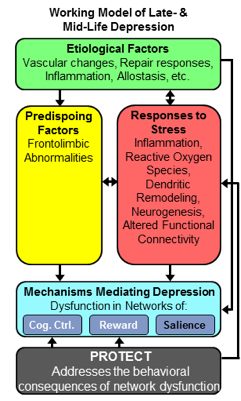
Elder mistreatment afflicts up to 10% of adults 60 years and older. Approximately 1/3 of victims have clinically significant depressive symptoms. We developed EM/PROTECT, a behavioral intervention for depressed EM victims to work in synergy with EM mistreatment resolution services that provide safety planning, support services, and linkages to legal services. PROTECT is built on a model, which postulates that chronic stress promotes dysfunction of the cognitive control (CCN) and the reward networks impairing the victims’ ability to flexibly respond to the environment and limits their rewarding activities. EM/PROTECT utilizes simple to use mobile technology to support the victim’s action plans, help therapists to target their sessions, and track mood, stress and social interaction. EM/PROTECT has been designed in an iterative process with community EM providers of the NYC Department for the Aging (DFTA) to use agencies’ routine PHQ-9 depression screening and referral for services.
This developmental project will examine the reach, feasibility, acceptability, and preliminary effectiveness of EM/PROTECT with the goal of building a sustainable mental health program that can be broadly implemented. Our primary hypotheses postulate that victims who receive EM services and PROTECT therapy (EM/PROTECT) will have greater reduction in depression and improvement in quality of life compared to victims who receive EM Services and a mental health referral (EM-MH). We propose that reductions in stress will mediate the differential impact of EM/PROTECT.
With two NYC EM agencies, we designed procedures for PHQ-9 screening, hand-off, linkages, consent process, tracking of EM actions, and clinical progress. EM victims are recruited from two randomly assigned EM agencies. Research assessments are conducted at baseline, 6, 9 and 12 weeks to evaluate depression, quality of life, chronic stress, rewarding activities and incidents of financial, physical and psychological abuse and neglect. We are assessing reach by recording the rates of screening, identified depressed victims, referrals to each condition, refusal and lack of follow-through; feasibility of EM/PROTECT based on drop-out, completion of assessments, and use of smartphones; and acceptability based on client satisfaction.
The results of this project will be used to support an R application to formally test the feasibility, acceptability, effectiveness, non-billable costs, and barriers to implementation of PROTECT in in a rigorous randomized controlled trial. The EM/PROTECT project is further described in our recent publication (http://www.ncbi.nlm.nih.gov/pubmed/25611116). For more information on the PROTECT program, please call Dr. Sirey and the PROTECT team at 914-997-4333.
ALACRITY: Developmental Project REDS
In response to the large numbers of senior center clients who suffer from untreated depression, we have partnered with the NYC Department for the Aging (DFTA) to develop SMART-MH, a community care model that can be embedded in senior centers to improve recognition, referral, and adherence to depression treatment. We also developed and tested Engage, a stepped-care therapy streamlined to use “reward exposure” as its principal intervention based on the assumption that dysfunction of the reward networks is central to the pathogenesis of depression. With our senior center partners and our mobile technology team, we redesigned Engage-M so that it can be used in a group format by licensed clinical social workers (LCSWs) of Senior Centers. Mobile technology provides probes for client adherence and offers to therapists easy to review summary records of mood, activity, and social interaction that can be used to target their sessions. We have integrated SMART-MH and Engage-M into a comprehensive community care model “Reaching and Engaging Depressed Senior Center Clients” (REDS).
This developmental project is studying the reach, feasibility, and acceptability of REDS and examines engagement of behavioral targets and preliminary effectiveness. We also collect information on REDS cost, barriers to implementation, and potential savings in health care utilization. Participants of four senior centers are randomly assigned to Engage-M (N=40), the treatment offered by REDS (1 individual and 8 weekly group sessions) or 8 group sessions “Wellness in Mind and Body” plus mental health referral (W-MH; N=20). The participants have clinically significant depressive symptoms (PHQ-9>10) and are older and middle-aged adults. Clients are identified by senior center staff trained in SMART-MH strategies. We offer additional training to staff of all centers on SMART-MH outreach, depression screening, and treatment engagement. Our findings will set be used in a R study to formally test the feasibility, acceptability, effectiveness, non-billable costs, and barriers of REDS beyond NYC. If proven effective, with administrative support, REDS can be extended nationally and supported by billable services.
Engage: A Novel Neurobiology Based Psychotherapy
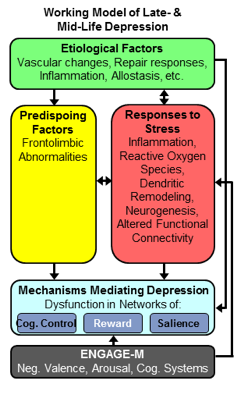
Engage is a novel psychotherapy for late life depression supported by a grant of the National Institute of Mental Health. It is based on neurobiological findings showing that abnormal function of the reward system promotes depression. Accordingly, Engage therapists help patients through a variety of behavioral interventions to enrich their daily experiences with meaningful and rewarding activities intended to reactivate their reward system. Participants to Engage are older adults with major depression.
The study is supported by a grant of the National Institute of Mental Health and offers a detailed clinical evaluation, brain MRI, and 9 weekly sessions of either Engage or Problem Solving Therapy, an evidenced based psychotherapy. The principal investigator, G. Alexopoulos, MD and the Engage research team can be contacted at (914) 997 4331.
Psychotherapy for Depressed Stroke Patients
Ecosystem Focused Therapy (EFT) is a novel psychotherapy designed to help depressed stroke patients by reducing their stressful experiences. It is based on findings indicating that the neurobiological responses to stress promote a multitude of processes leading to depression. To interrupt these processes, EFT therapists apply a variety of techniques aiming to enable patients to develop adaptation skills and a perspective useful for adjusting to their disability. EFT therapists also work with the patients’ family and specialized therapist to develop a human and physical environment (ecosystem) that best accommodates the patients’ new disability. Thus, EFT offers an optimal chance for adaptation, by promoting the patients’ adherence to rehabilitation and by increasing their sense of competence and their engagement in activities they value.
The study is supported by a grant of the National Institute of Mental Health and offers detailed clinical evaluation and sessions of either EFT or a psychoeducation therapy. The principal investigator, G. Alexopoulos, MD and the research team can be contacted at (914) 997 4331.
Sandy Mobilization, Assessment, Referral and Treatment for Mental Health (SMART-MH)
The Sandy Mobilization, Assessment, Referral and Treatment for Mental Health (SMART-MH) program is designed to identify the mental health and social support needs of older adults in areas impacted by Superstorm Sandy. The SMART-MH model integrates community outreach, detection of mental health need and community delivery of psychotherapy for depression and anxiety. SMART-MH is community based and targets people ages 60 and older living in areas impacted by Hurricane Sandy. SMART-MH uses an evidence-based approach to assessment, interventions and psychotherapy to evaluate and to address both specific mental health needs (e.g., depression, anxiety, alcohol abuse) and the impact of the storm. For information on SMART-MH, please contact Dr. Sirey and the SMART-MH team at 212-821-0759.
Treatment Initiation and Participation Program (TIP)
Antidepressant nonadherence remains a significant challenge to effective depression treatment offered to older adults in primary care settings. The Treatment Initiation and Participation program, TIP is a brief psychosocial intervention to improve adherence to pharmacotherapy. The intervention was designed to address the individual level barriers to care that are associated with medication nonadherence and treatment drop out such as stigma, worries about medication side effects, and difficulty developing an adherence strategy. To learn more about TIP, please call Dr. Sirey at 914-997-4333.
Trio for Successful Aging (Trio for short)
This program extends the SMART-MH model to other aging service sites. It brings together mental health and medical expertise in an integrated program for community older adults delivered in senior centers that can provide social and aging support services. A fundamental tenet of this program is that effective treatment of the mental health needs of older adults also requires us to understand and address their physical and social health needs, which often trigger and perpetuate mental health problems in later life. For more information on the TRIO program, please call Dr. Sirey at 212-821-0759 or 914-997-4333.
Psychotherapies for Suicide Prevention
Suicide rates in middle-aged and older adults are alarmingly high. We have developed psychosocial interventions to reduce suicide risk and at risk middle-aged and older adults. We are recruiting middle-aged and older outpatients with suicidal ideation and treatment resistant major depression and inpatients who have been hospitalized for suicidal ideation or suicide attempt. Our therapies are based on our theory that maladaptive emotion regulation is associated with increased suicide ideation and suicidal behavior. Therefore, therapists teach the patients emotion regulation techniques to help reduce their suicide risk. The principal investigator, Dr. Kiosses and his research team can be contacted at (914) 997 4331. For more information, please look at Dr. Kiosses’ research webpage.
Psychotherapy for Depression and Cognitive Impairment
Problem Adaptation Therapy (PATH) is a new psychotherapy that utilizes emotion regulation techniques based on Dr. James Gross’ process model of emotion regulation. PATH utilizes compensatory strategies and environmental adaptations to bypass cognitive and functional limitations in older adults with depression and cognitive deficits and invites the participation of caregivers when necessary. The principal investigator, Dr. Kiosses, and his research team can be contacted at (914) 997 4381. For more information, please visit Dr. Kiosses’ research webpage.
Project EVO for Late-Life Depression
The purpose of this study is to test an IPad-delivered cognitive intervention for middle-aged and older adults who suffer from depression and have difficulty with executive skills. Executive skills are a group of mental processes that include the ability to concentrate, multitask, and organize. The intervention is performed on an IPad for 20 minutes per day/5 days per week over a four-week period. Participants also will see a coach/study therapist once per week during the study. We are investigating the impact of the cognitive intervention on executive skills, depressed mood, and brain activation assessed by magnetic resonance imaging (MRI). Participants should be between the ages of 45 and 75 years and depressed at the time of enrollment in the study. To find out more information, please contact us at 914-997-4331.
Antidepressant Medication for Patients with Depression
The purpose of this study is to identify how problems with the brain’s ability to ignore distractions and control emotions may lead to or complicate depression in older adults. This research is being done to help develop tests that may better detect older adults at risk for this type of depression and to improve their treatment outcomes. Eligible participants receive an magnetic resonance imaging (MRI) and 12 weeks of the FDA approved antidepressant medication escitalopram under the supervision of a psychiatrist. This study is funded by the National Institute of Mental Health. The principal investigator, Faith Gunning, Ph.D., and the treatment team can be reached at 914-997-4331.


